Researchers have discovered that physically squeezed cancer cells rapidly deploy mitochondria to the nucleus, creating a power-boosting 'halo' that fuels DNA repair and promotes survival, a finding that opens new avenues for anti-metastasis therapies.

October 5, 2025

Source:
Drug Target Review
A Cellular Emergency Power Grid
Scientists have uncovered a startling survival mechanism in cancer cells: when physically squeezed, they instantly reroute their power source to protect their genetic core. In a process visualized for the first time, mitochondria—the cell's powerhouses—rush to the nucleus to form a temporary halo-like structure.
These structures, dubbed nucleus-associated mitochondria (NAMs), act as an emergency power grid. They deliver a massive burst of ATP, the main cellular energy molecule, directly to the nucleus. This immediate power surge fuels DNA repair, allowing the cell to withstand the physical stress encountered during tumor growth and spread.
This discovery highlights a new layer of metabolic plasticity in cancer, a key focus in oncology research as detailed in reviews like those in Nature Reviews Cancer. The findings show how cancer cells dynamically manage energy to survive threats.
Keep up with the story. Subscribe to the PR+ free daily newsletter
Source:
SciTechDaily
Visualizing a Survival Tactic
Using advanced live-cell microscopy, researchers watched this cellular drama unfold in real time. The formation of NAMs is an incredibly rapid response to physical confinement.
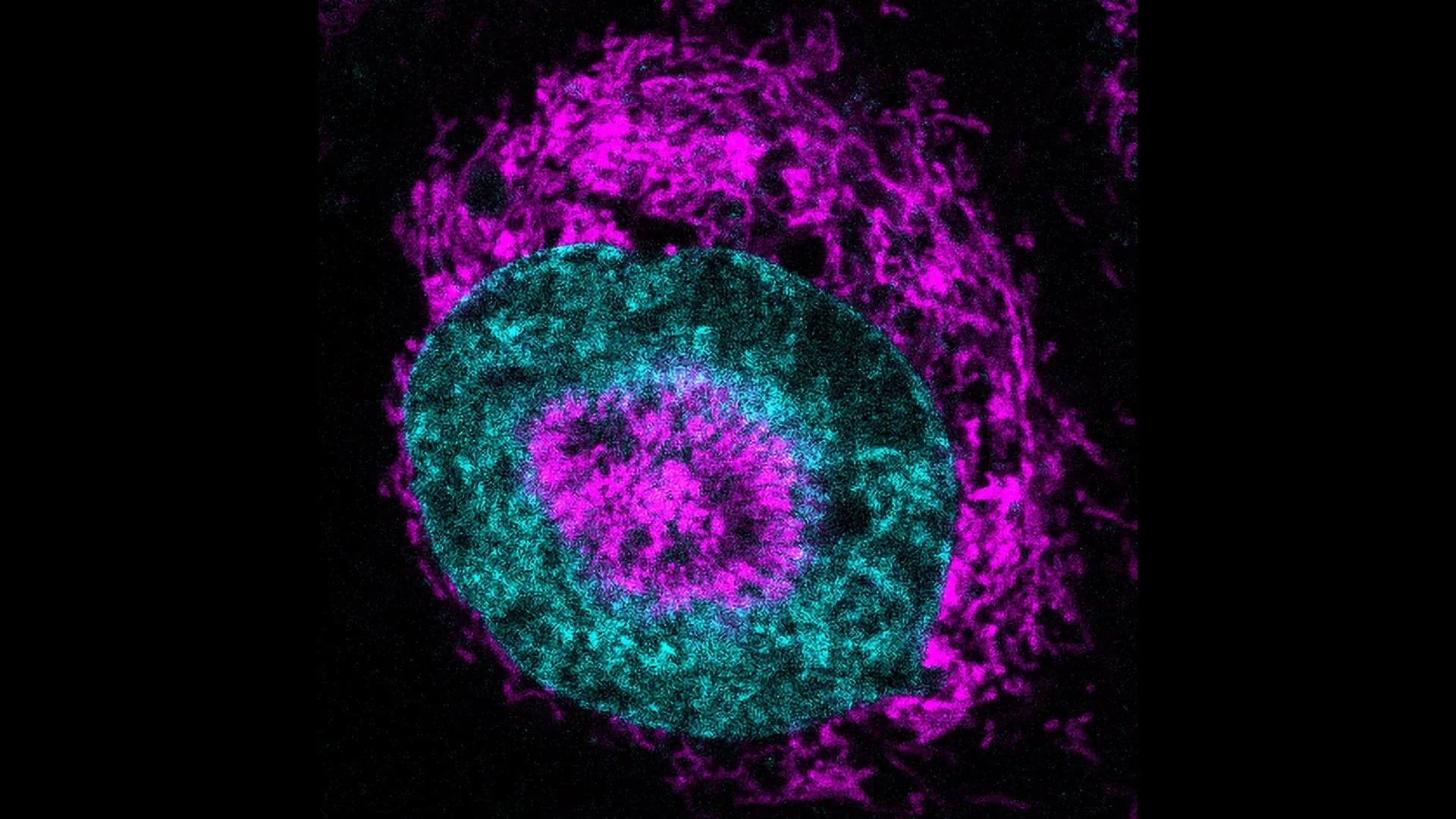
The 'Mitochondrial Halo'
Within seconds of being compressed, mitochondria in HeLa cancer cells were observed clustering tightly around the nucleus. This "halo" is held in place by the cell's internal scaffolding, primarily a mesh of actin filaments. This structure effectively traps the mitochondria, ensuring a targeted energy delivery system.
Evidence in Human Tumors
The significance of this mechanism was confirmed in human tissue. Biopsies from 17 breast cancer patients revealed that NAMs were three times more frequent at the invasive edges of tumors than in the less active core. This suggests that NAMs are not just a laboratory phenomenon but a critical tool for cancer cells as they invade surrounding tissue, a hallmark of metastasis.
Read More
Source:
ScienceDaily
Share this news:




















